NeuroStar Transcranial Magnetic Stimulation
TMS Center of Wisconsin
Get immediate answers to any other questions you may have
What is Transcranial Magnetic Stimulation?
Since the 1980s, transcranial magnetic stimulation has been used to study the nerve fibers that carry information about movements from the brain to the spinal cord and on to the muscles.
In the late 1990s, physicians began to explore the therapeutic potential of transcranial magnetic stimulation for the treatment of a variety of diseases, with depression being the most thoroughly studied to date. Since then, more than 20 randomized, controlled trials studying transcranial magnetic stimulation as a treatment for depression have been published by investigators throughout the world.
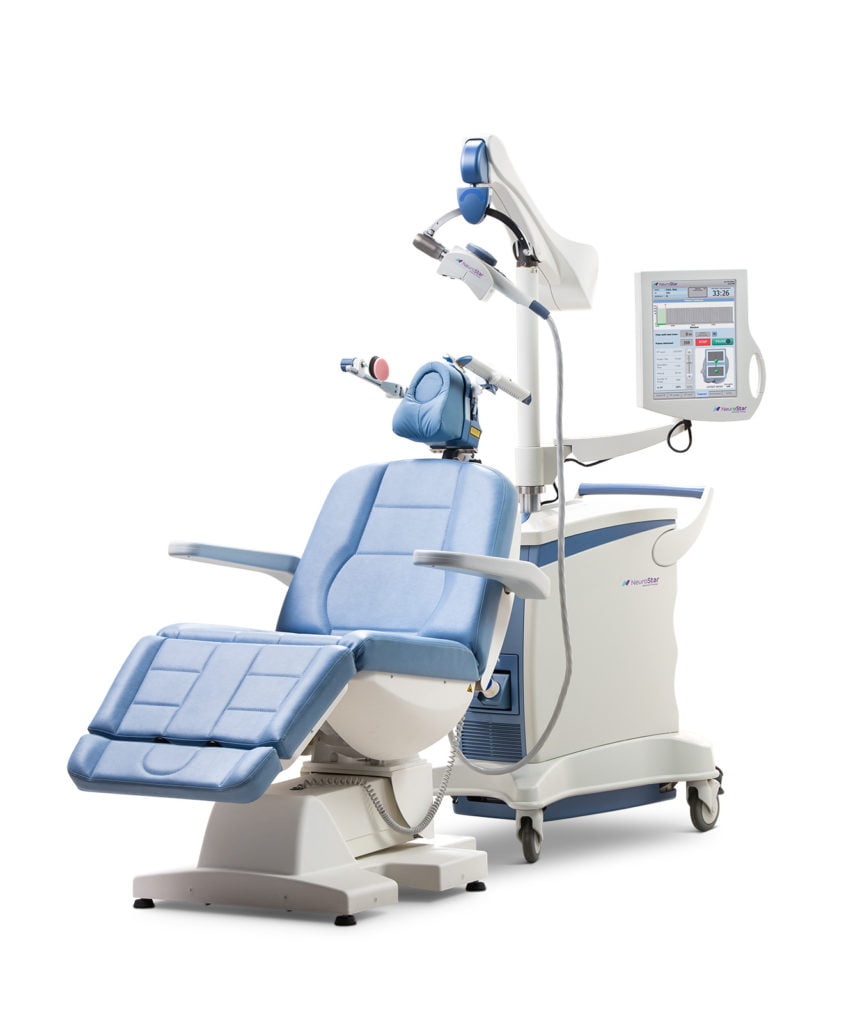
NeuroStar TMS (transcranial magnetic stimulation) Therapy was FDA-cleared in October 2008 for patients suffering from depression who have not achieved satisfactory improvement from prior antidepressant medications. Using pulsed magnetic fields, transcranial magnetic stimulation therapy stimulates the part of the brain thought to be involved with mood regulation.
TMS Therapy is a short outpatient procedure, performed in your psychiatrist’s office under his or her supervision while you remain awake and alert. The typical initial course of treatment is about 19-37 minutes, depending on what the doctor determines is the correct protocol, daily over 4-6 weeks.
TMS Center of Wisconsin
Get immediate answers to any other questions you may have
About NeuroStar TMS Therapy
NeuroStar TMS Therapy is:
- Non-invasive, meaning that it does not involve surgery. It does not require any anesthesia or sedation, as the patient remains awake and alert during the treatment.
- Non-systemic, meaning that it is not taken by mouth and does not circulate in the bloodstream throughout the body.
A Typical TMS Treatment Procedure
A NeuroStar® TMS Therapy treatment session is a short outpatient procedure that lasts about 19-37 minutes, depending on what the doctor determines is the correct protocol. During treatment, you can relax in the treatment chair. You can also speak with our TMS Specialists whenever necessary. After the procedure, you can immediately return to your normal routine, including driving.
Your first treatment session
Because your TMS-certified physician needs to determine how to most effectively administer treatment, your first session could last up to an hour and a half. You will be provided and asked to wear protective earplugs, as the system emits a tapping sound during operation.
Your TMS physician will first perform a test to identify your motor threshold. The motor threshold is the amount of magnetic field strength that results in a movement of your right thumb. This test is important because it identifies the magnetic field strength that will be used in your treatment. This field strength is customized for each patient to deliver the correct treatment dose.
After this initial procedure, the TMS physician will determine the place on the head where the TMS treatment will be applied and the magnetic coil will be moved to that location. This will allow you to receive optimal treatment.
The TMS Specialist will then administer TMS Therapy over an approximately 19-40 minute period. In 30-second intervals, the device will deliver rapid “pulses” of the magnetic fields. These will feel like tapping on your scalp. Some patients may find this tapping uncomfortable. Your physician may be able to make adjustments to reduce this discomfort.
After the procedure
Immediately following each treatment session, you may return to your normal daily routine, including driving. During or after treatment you may experience headache or discomfort at the site of stimulation. These are common side effects that often improve as further treatment sessions are administered.
If necessary, you can treat this discomfort with an over-the-counter analgesic. If these side effects persist, your TMS physician can temporarily reduce the strength of the magnetic field pulses being administered in order to make treatment more comfortable.
In clinical trials, most patients who benefited from NeuroStar TMS Therapy experienced results by the fourth week of treatment. Some patients may experience results in less time, while others may take longer. You should discuss your depression symptoms with your physician throughout the treatment course. If symptoms persist, you may want to consider other antidepressant options.
TMS Therapy Insurance Coverage
Since the FDA clearance of TMS in 2008, insurance coverage for eligible patients has increased significantly. Currently, there are over 60 coverage polices for TMS, including most Medicare contractors. Although TMS is not a first line of treatment, it is an alternative option for those who are not responding to or cannot tolerate medications.
Patients are encouraged to speak directly with their Doctor regarding any specific insurance questions. Patients can also contact a Reimbursement Specialist and receive assistance with understanding insurance coverage and verifying insurance benefits. For a list of the insurance companies and applicable states that cover TMS Therapy, please click here.
TMS Center of Wisconsin
Get immediate answers to any other questions you may have

How Does TMS Work?


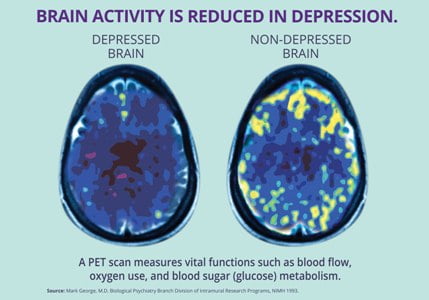
Through a magnetic coil, the NeuroStar TMS Therapy system generates highly concentrated, magnetic fields which turn on and off very rapidly. These magnetic fields are the same type and strength as those produced by a magnetic resonance imaging (MRI) machine.
The treatment coil is applied to the head above the left prefrontal cortex. This part of the brain is involved with mood regulation, and therefore is the location where the magnetic fields are focused.
These magnetic fields do not directly affect the whole brain; they only reach about 2-3 centimeters into the brain directly beneath the treatment coil. As these magnetic fields move into the brain, they produce very small electrical currents.
These electrical currents activate cells within the brain which are thought to release neurotransmitters like serotonin, norepinephrine, and dopamine. Since depression is thought to be the result of an imbalance of these chemicals in the brain, TMS can restore that balance and, thus, relieve depression.
Neurostar® Effectiveness
Clinical trials have demonstrated the effectiveness of NeuroStar® TMS Therapy in treating patients who have not benefited from prior antidepressant medication. NeuroStar TMS Therapy was studied in adult patients suffering from Major Depressive Disorder, all of whom had not received satisfactory improvement with previous treatments.
An Effective and Durable Option for Treating Major Depressive Disorder.
In an independent, randomized, controlled trial funded by the National Institute of Mental Health, 307 patients were treated with the NeuroStar TMS System for 4 to 6 weeks, similar to real clinical context.
Patients were divided into two groups:
Low Treatment Resistance: Patients who have failed to improve their depression symptoms after a single antidepressant treatment of adequate dose and duration.
High Treatment Resistance: Patients who have failed to improve their depression symptoms after a multiple (2-14) antidepressant treatments of adequate dose and duration.
At the end of their treatments, patients who had received NeuroStar TMS Therapy were four times more likely to achieve remission compared to patients receiving a sham treatment. 1 in 2 patients experienced significant improvement in their depression symptoms and 1 in 3 experienced complete remission.
Patients treated with the NeuroStar TMS System also experienced significant improvement in anxiety and physical symptoms (such as appetite changes, aches and pains, and lack of energy) associated with depression.
Durability of TMS Treatments
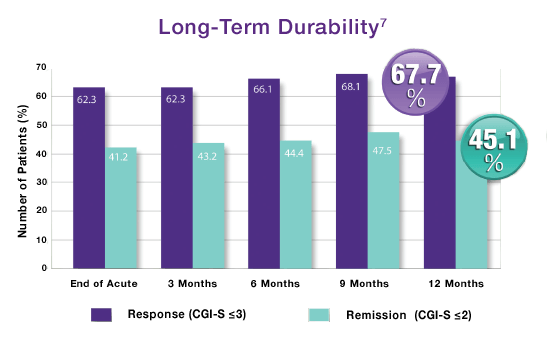
NeuroStar TMS is the only TMS system with the durability of its effects established over 12 months.
In a trial with physician directed standard of care, meaning Neurostar TMS Therapy could be used in conjunction with antidepressants as needed, patients who had received treatment then reported their symptom levels at 3, 6, 9 and 12 months to determine the durability of their treatments.
By the end of the 12-month period, 2 out of 3 patients who had either responded or completely remitted after TMS treatment remained at the symptom levels they reported at the end of the treatment phase.1
After the end of the treatment period, only 1 in 3 patients needed to come back for maintenance TMS sessions, or ‘reintroduction’ during this 12-month period.
Treatment Algorithm
Additionally, Neuronetics has developed a new tool to use in educating patients on the best practices of treating depression. The Best Practices Treatment Guideline for Depression has been developed to help patients understand TMS Therapy as an option if their first line antidepressant medications stop working.
This guideline is based on the 2010 American Psychiatric Association’s practice guidelines and NeuroStar TMS Therapy indication for use, which says:
NeuroStar TMS Therapy is indicated for the treatment of major depressive disorder in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication at or above the minimal effective dose and duration in the current episode.
Please see the Treatment Algorithm for an effective illustration of the use of NeuroStar TMS Therapy early on in the treatment of depression.
NeuroStar TMS Therapy has not been studied in patients who have not received prior antidepressant treatment.
TMS Center of Wisconsin
Wisconsin’s First and Most Experienced TMS Center!
Neurostar® Safety
TMS Center of Wisconsin
Get immediate answers to any other questions you may have
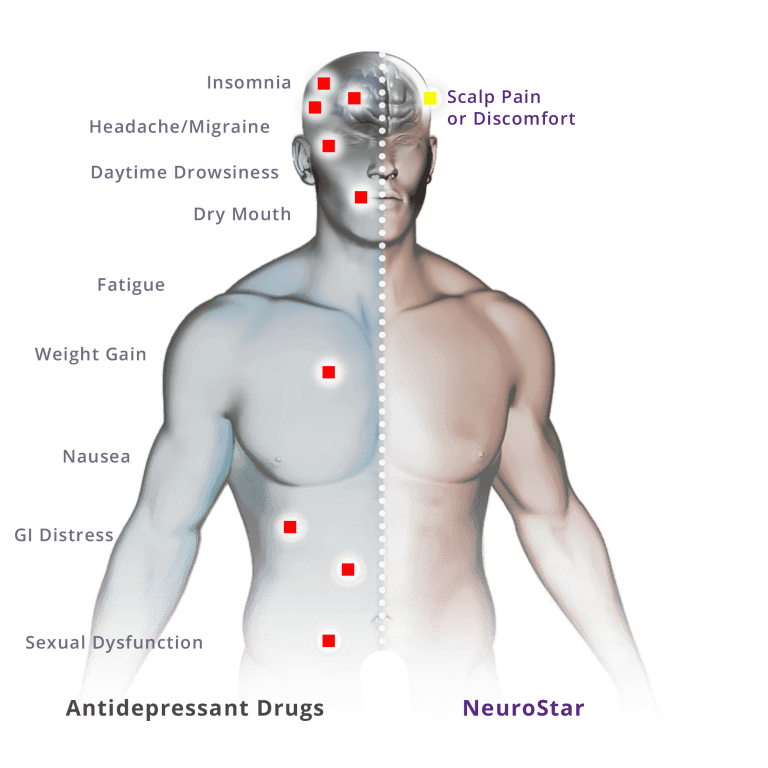
Clinical trials have demonstrated the safety of NeuroStar TMS Therapy® in treating patients who have had an inadequate response to prior antidepressant medications.
Treatment with NeuroStar TMS Therapy caused very few side effects and was generally well tolerated by patients. The most common side effect reported during clinical trials was scalp discomfort—generally mild to moderate and occurring less frequently after the first week of treatment.
Fewer than 5% of patients discontinued treatment with NeuroStar TMS Therapy due to adverse events.
Over 10,000 active treatments were performed across all NeuroStar® clinical trials demonstrating its safety1
1. No systemic side effects
- No weight gain
- No sexual dysfunction
- No sedation
- No nausea
- No dry mouth
2. No adverse effects on concentration or memory
3. No drug interactions
4. NeuroStar TMS Therapy should not be used in patients with implanted metallic devices or non-removable metallic objects in or around the head. This does not include metallic fillings in teeth.
5. NeuroStar TMS Therapy should not be used in patients with implants controlled by physiological signals. This includes pacemakers, implantable cardioverter defibrillators (ICDs), and vagus nerve stimulators (VNS).
Learn about a typical NeuroStar TMS Therapy treatment session.
BOOK CONSULTATION
Contact Info
Give us a call to discuss your treatment options
